Counterfeit Botox is a real danger. Here’s how to avoid it.
| ABCS
The Food and Drug Administration (FDA) issued a public warning in April 2024 that counterfeit Botox® was used in multiple states and led to several patient complications, including “blurred or double vision, difficulty swallowing, dry mouth, constipation, incontinence, shortness of breath, weakness and difficulty lifting one’s head.”
Below, the American Board of Cosmetic Surgery explains how to avoid dangerous black-market Botox products.
Botox® packaging: Legitimate vs. counterfeit
The FDA shared photos of the counterfeit Botox packaging, which would appear convincing to a provider or patient not already familiar with real Botox packaging.
Real Botox packaging and vials
Botox is manufactured by Allergan Aesthetics (AbbVie). Real Botox packaging has an “Allergan” hologram over the label and says “OnabotulinumtoxinA” in a purple banner across the top.
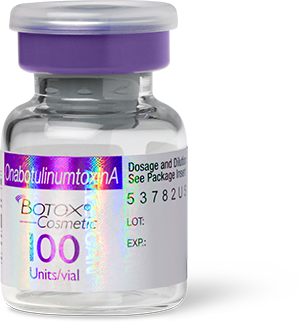
Image Source: The U.S. Food and Drug Administration.
Counterfeit Botox packaging and vials
The recently discovered counterfeit Botox packaging displayed a firework-pattern hologram and lot number C3709C3. The vial shows no hologram.



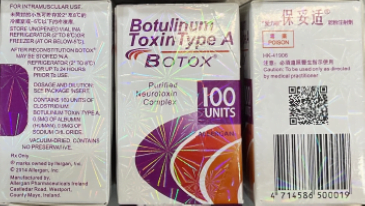
Image Source: The U.S. Food and Drug Administration.
There are only 5 FDA-approved neuromodulators
The FDA requires a stringent approval process for all prescription medications, which includes neuromodulators like Botox. There are five total Botox-like injectables that are currently approved for use in the United States:
- Botox®
- Dysport®
- Jeuveau®
- Xeomin®
- Daxxify™
Patients should feel comfortable asking their provider which brand of neuromodulator they are receiving, as many providers offer multiple options. A physician (MD or DO) is required to purchase these products and should oversee all injections and treatment plans, even in medical spas or practices where a Physician Assistant or Registered Nurse performs injections.
Established providers source their medications from trusted representatives of the manufacturer
While it remains unclear who injected the fake Botox and where it was purchased, experienced cosmetic surgeons, dermatologists, and other physicians who oversee safe medical spas will have established relationships with the manufacturers of these FDA-approved injectables, and would be able to recognize counterfeit products.
References »
Counterfeit Version of Botox Found in Multiple States. Food and Drug Administration (FDA).
FDA Issues Alert on Counterfeit Botox: What Dermatology Clinicians Need to Know. Dermatology Times.
About the American Board of Cosmetic Surgery
The American Board of Cosmetic Surgery (ABCS) is a certifying board dedicated exclusively to excellence in cosmetic surgery. Our surgeons specialize in cosmetic enhancements and have passed rigorous exams and screening to illustrate their safety and aesthetic abilities, and many offer non-surgical medical spa treatments from their cosmetic surgery practices. Learn more about choosing a cosmetic surgeon here, or find a board-certified cosmetic surgeon near you in our surgeon directory.